Sign up for our newsletter!
Your data will be handled in compliance with our privacy policy.
Your data will be handled in compliance with our privacy policy.

Smolek has received patents for three brand-new innovations that all aim to improve the electrical contact inside PEM electrolyzers, fuel cells, and batteries. This article briefly explains the three innovations in layman’s terms. Hopefully, after reading, you will be as excited as we are about the unique position Smoltek has gained in the clean energy market with these patents.
It’s easy to receive news that Smoltek has been granted new patents with a shrug or a yawn. After all, such news comes along all the time. Usually, it is a previously known innovation that has been granted a patent in yet another country. The umpteenth in a row. This is, of course, good. But not necessarily something to write home about. So you’re forgiven if you missed the bombshell: Smoltek has been granted patents for three breakthrough innovations. We believe these inventions will make electrolyzer and fuel cell manufacturers crave our technology and know-how even more. Curious? Read on!
The transition to sustainable energy depends on the efficiency of electrochemical cells – the workhorses behind technologies such as fuel cells and electrolyzers. However, these essential components face two problems: high contact resistance and rapid corrosion.
High contact resistance leads to significant energy losses, resulting in reduced performance and increased operating costs.
Corrosion erodes the structural integrity of the electrochemical cells, causing them to wear out quickly and require replacement.
All manufacturers of fuel cells and electrolyzers want to tackle these two challenges. Better solutions are needed.
Guess who is sitting on them?
That’s right! Smoltek.
Our super-talented researchers and developers have invented not one but three solutions that work together to deal with these problems.
Smoltek’s three patents form a formidable arsenal against the dual challenges of high contact resistance and corrosion, marking a significant leap in electrochemical cell technology.
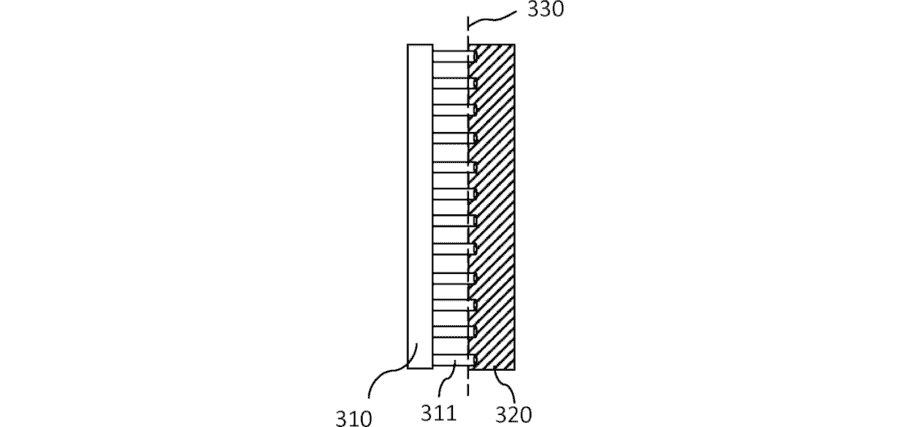
Patent SE545845 covers an innovation we have given the completely unimaginative name of ”a separator plate arrangement for an electrochemical cell comprising a nanostructure.” To avoid dropping dead of boredom when saying the name, we use the much sexier unofficial name: Contact Resistance. This is a misnomer, as the innovation is not about contact resistance but counteracting it. As the official name suggests, this is accomplished by arranging the flow plates in an electrochemical cell in a certain way. More about that later.
Patent SE545846 has a much cooler name: Nano Velcro. Sure, it’s not the official name; it’s as dry as the paper the patent is written on: “Fuel cell or electrolyzer with a connective nanostructure.” More information will follow on this as well. However, you are correct if you conclude from the official name that it is also about reducing contact resistance.
Patent SE545852 follows and covers also an innovation to lower contact resistance. (Resistance is futile!). The official name doesn’t give much away: “A separator element with a coating comprising nanostructures.” But our unofficial name is a bit more revealing: Vertical Graphene. It is similar to the first patent, but uses vertical graphene instead of carbon nanofibers. We’ll get to what that means.
Are you ready to explore the patents a bit further?
An electrochemical cell is where the magic happens in electrolyzers (electricity and water become hydrogen and oxygen) and fuel cells (hydrogen and oxygen become electricity and water). Thus, it’s a critical component in many applications needed if we are to transition from an energy system with tons of carbon emissions to a green and clean energy system.
A membrane is at the very core of an electrochemical cell. PEM electrolyzers and fuel cells use a membrane that lets protons through but blocks electrons, forcing them to detour through wires. This is what makes the magic possible.
However, to work, electrical contact between an electrode and the surface of the membrane is required. How hard can it be? Just press an electrode against the membrane, and it’s done.
Or is it?
Of course, it is not that simple. A lot of water and hydrogen gas must also fit in the interface between the electrode and the membrane, and if you create some space for it, the electrical contact is broken.
The way to resolve this is to have electrically conductive porous material between the electrode and the membrane. The pores allow water and hydrogen to pass through while providing pathways for the current to flow between the electrode and the membrane.
Easy, huh?
No. The difficulty is to create a good electrical contact between the electrically conductive porous material and the membrane. And prevent the contact surface from oxidizing because it is electrically insulating.
Enter the stage: Smoltek’s innovation.
If you have been a keen student, you will recognize the solution. We’ve made no secret of it since we applied for the patent. (Inventions become public at the time of application, but in return enjoy protection during the application period.)
Smoltek’s innovation is to insert nanofibers between the electrode and the membrane. These are attached to the electrode or porous material, and the tip is stuck into the membrane. Electrically conductive carbon nanofibers in electrical contact with the electrode and membrane reduce contact resistance.
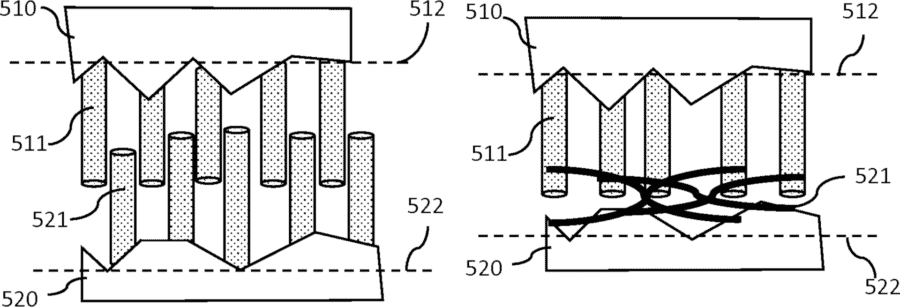
Do you like Seinfeld? The TV series? I love it. And like many other fans of television’s greatest shows of all time (that’s a scientific fact), I can see parallels and similarities everywhere between life and the show. In this case, when we talk about Nano Velcro, it should be pretty obvious that I’m thinking of Barney Martin’s line, as Morty Seinfeldt: ”I can’t stand velcro. That tearing sound.” Unlike Morty, we love velcro, at least if they are made of carbon nanofibers and are used to create strong electrical contact between two layers in an electrochemical cell.
For example, let’s return to the contact surface between an electrically conductive porous material and the surface of a membrane. Current technology is to press them together and hope that there is enough electrical contact. But this has many problems.
On the scale that electrons move, there are no smooth surfaces in perfect contact with each other. Irregularities and growing oxidation make the contact surface smaller than you might think. There is also a lot of water and gases flowing, causing the surfaces to vibrate, further deteriorating the contact.
In short, it is almost a miracle that today’s PEM electrolyzers and fuel cells even work.
This is where our Nano Velcro comes in. The idea is to grow carbon nanofibers from both surfaces and allow them to be mechanically entangled together, much like a piece of velcro. These carbon nanofibers should neither be completely straight nor grow straight out from the surface, but they should twist a little and grow at a slight angle. This increases the entanglement when they are pressed together. One can even let the carbon nanofibers on one side grow almost parallel to the plane they are attached to, to effectively create mechanical interlocking.
This creates larger contact surfaces; each carbon nanofiber has a surface area orders of magnitude larger than the surface it grows on. In addition, the entanglement creates a mechanically more stable contact.
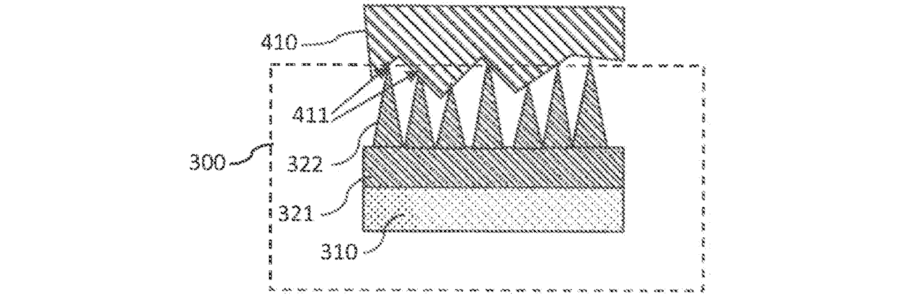
This innovation aims at the same thing as the other two: creating larger and more stable contact surfaces between an electrically conductive porous material and the surface of a membrane. Just like in the first-mentioned patent (SE545845), nanostructures protruding into the membrane do the trick. But unlike that, it’s not carbon nanofibers but vertical graphene that does the job.
Carbon atoms can bond to each other in many different ways, creating many different structures. Graphene is one of them.
In graphene, the carbon atoms form hexagonal rings, where adjacent rings share sides and are in the same plane. It looks like a sheet of chicken wire where the knots are carbon atoms, and the threads between them are their bonds.
While the “regular” graphene chicken wire grows along the substrate, vertical graphene grows perpendicular to the substrate. They form a chicken wire fence that twists and turns. It looks like a curved wall. This is why vertical graphene is also called carbon nanowalls. They are criss-crossing and highly interconnected.
So what’s the point of replacing the well-known and trusted carbon nanofibers with the newcomer, vertical graphene? Here’s the tri: this fresh entrant in the nano-world is like carbon nanofibers on steroids. All the things that carbon nanofibers do well, they do better:
In other words, these new kids aren’t just matching the old boys – they’re outplaying them on many fronts.
I have tried to describe the innovations covered by the patents in a hopefully understandable way. Therefore, I have had to simplify the description and focus on the most immediate application areas (fuel cells and PEM electrolyzers). Therefore, this description does not do full justice to the scope and applications of the innovations.
What I mean by this is that the patents protect far more than just improved electrical contact between electrode and proton exchange membrane with a porous transport layer in between; they protect far more electrochemical cells than fuel cells and PEM electrolyzers. For example, they also protect the anion exchange membrane (AEM), which may be used in future electrolyzers and various forms of batteries.
My point is that these three patents give Smoltek the exclusive right for twenty years to dictate the conditions for using these types of solutions in all possible contexts.
Life goes on. We continue to develop our cell material for PEM electrolyzers.
In the case of the three patents, they each form a new patent family. As the patents for the three innovations are approved in the EU, the US and other countries, the new patent families will grow.
For Smoltek’s shareholders and investors, these patents are more than just a testament to Smoltek’s unique expertise and inventiveness; they represent a true strategic advantage in the growing clean energy market.
Our company possesses technology and know-how that is increasingly sought after as the demand for clean energy solutions grows. We can safely say that Smoltek is very well poised to face the future and become a key player in the field.
Don’t you agree?
Your data will be handled in compliance with our privacy policy.