Sign up for our newsletter!
Your data will be handled in compliance with our privacy policy.
Your data will be handled in compliance with our privacy policy.
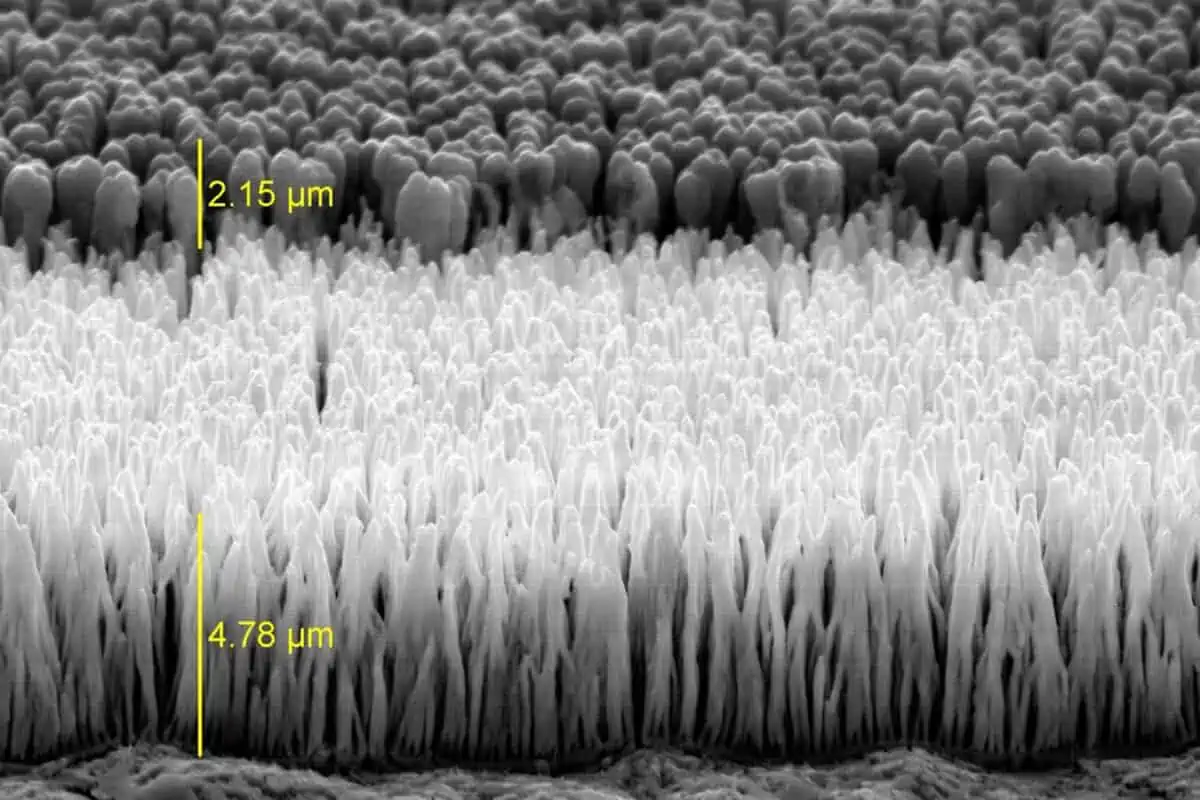
Smoltek’s patent-protected technology platform enables controlled growth of precisely localized and defined nanostructures, as individual fibers or clusters, in predefined patterns or films. This is done in a through catalytic growth in a vacuum chamber using gas and catalysts. Materials and process conditions are compatible with industrial requirements.
We have developed unique growth recipes using which we can grow carbon nanostructures at exact positions with exact required properties. This is our core technology, and it goes by the name SmolGROW™. Partners can license our technology to accomplish solutions tailored to their unique needs and requirements.
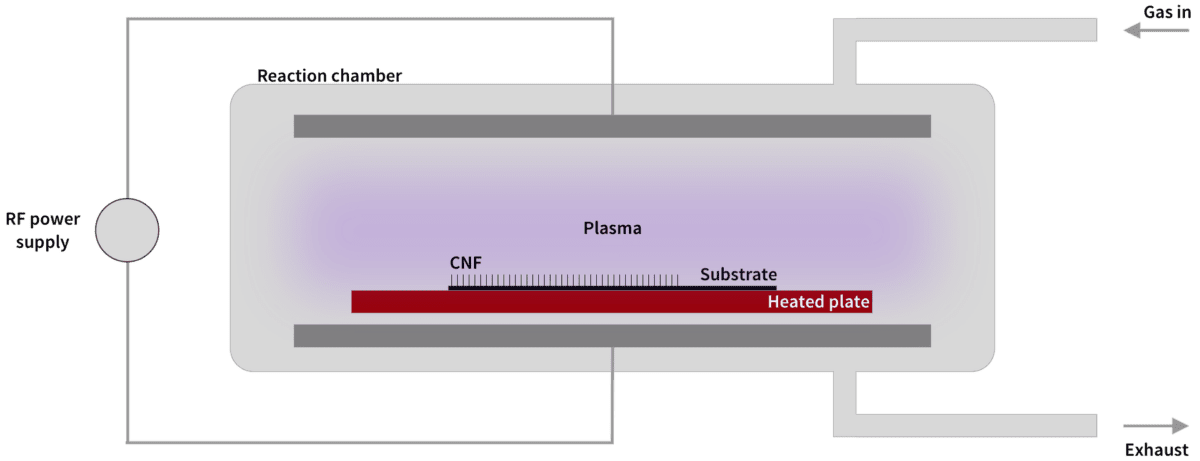
Smolek’s fabrication of carbon nanofibers is done with Plasma Enhanded Chemical Vapor Deposition (PECVD). In this process we apply energy to a carbon-based gas, in the form of heating or lightning discharges, releasing carbon ions that can deposit on surfaces prepared with catalytic metal.
In our SmolGROW™ process we can exactly control where the released carbon atoms settle and how they are formed into carbon nanofibers (or nanostructures). This gives us a unique opportunity to tailor carbon nanofibers with desired properties. We have also developed the technology to use comparatively low temperatures (375 °C), which allows our manufacturing technique to be used in production lines in the semiconductor industry.
The SmolGROW™ CNF manufacturing process (overview):

Smoltek’s technology works not only with carbon nanofibers. It is possible to create other carbon nanostructures, like carbon nanotubes (CNTs), and use materials other than carbon.
Learn more about carbon nanotechnology from the articles below, or contact us and let’s have a chat.
With our carbon nanofibers (CNFs) fabrication technology, we develop advanced materials engineering solutions for use in water electrolysis and fuel cells for the hydrogen industry.
With our carbon nanofibers (CNFs) fabrication technology, we develop advanced packing solutions and ultra-miniaturized capacitors for use in the semiconductor industry.
Smoltek has developed the world’s thinnest discrete capacitor. You have to stack ten of them on top of each other to reach the same height as today’s industry-standard when it comes to surface-mounted capacitors. The most amazing thing about this microscopic capacitor is its performance. One square millimeter has a capacitance of a whopping 650 nanofarads (650 nF/mm2). Read on for more details.
Carbon nanofibers in hydrogen electrolysis & fuel cells Hydrogen has emerged as a key to store renewable energy and making heavy industry carbon-free. Two application areas of immediate vital importance. The core technologies that make this possible are hydrogen electrolysis and fuel cells. Electrolysis converts electricity into hydrogen, while fuel cells convert the hydrogen back to electricity.