Sign up for our newsletter!
Your data will be handled in compliance with our privacy policy.
Your data will be handled in compliance with our privacy policy.

With our carbon nanofibers (CNFs) fabrication technology, we develop advanced materials engineering solutions for use in water electrolysis and fuel cells for the hydrogen industry.
The demand for sustainably produced hydrogen is rising due to its role in avoiding greenhouse gas emissions. Production and use of sustainable hydrogen is made possible by two core technologies: Water electrolysis, which produces hydrogen from water using electricity, and fuel cells, which reverses the reaction to generate electricity. However, both technologies use rare and expensive catalyst materials such as platinum or iridium. Using carbon nanofibers (CNF) as a catalyst support can decrease the amount of expensive catalyst material needed. Smoltek nanostructure fabrication technology can unlock this potential.
Hydrogen produced by water electrolysis can be a solution to several problems in the process of reducing greenhouse gas emissions. For example, intermittent energy sources such as solar and wind power occasionally produce more electricity than needed, e.g. on very windy or sunny days.
Using this electrical power for water electrolysis allows the energy to be stored in the form of produced hydrogen gas, which can later be used converted back to electricity by a fuel cell forming only water vapor in the process. Another key driver is to abate the carbon dioxide emission in the production of steel, where coal and coke can be replaced by hydrogen gas as reducing agent for the iron ore emitting only water vapor and making a significant reduction in global carbon dioxide emissions possible. This enables a fossil-free steel production.

Water electrolyzers use two electrodes, a positively charged anode and a negatively charged cathode, separated by an electrolyte that allows ions to travel between the electrodes. The electrodes are also electrically connected to a power source that supplies electrical power to drive the reaction. Oxygen gas is produced at the anode through the oxygen evolution reaction, and hydrogen gas is produced at the cathode through the hydrogen evolution reaction. Catalysts are used to promote these electrochemical reactions and allow them to run at a lower energy cost. The reactions happen at the active catalyst surface, i.e. at surfaces where the catalyst is in contact with the electrolyte.
Beneficial conditions for water electrolysis depend on the type of electrolyte, the operating temperature, the pressure and the types of catalysts. Historically the industry has mostly relied on low-temperature electrolyzers using an alkaline solution with high pH containing potassium hydroxide in water. They do not require scarce catalyst material but cause limited current densities and therefore limited hydrogen output per cell area. This drawback can be overcome by polymer ionomer membranes that conduct either hydrogen ions or hydroxide ions in direct contact with anode and cathode, so-called zero-gap design. These ion-conducting polymers are known as proton exchange membranes (PEM) or anion exchange membranes (AEM) respectively.
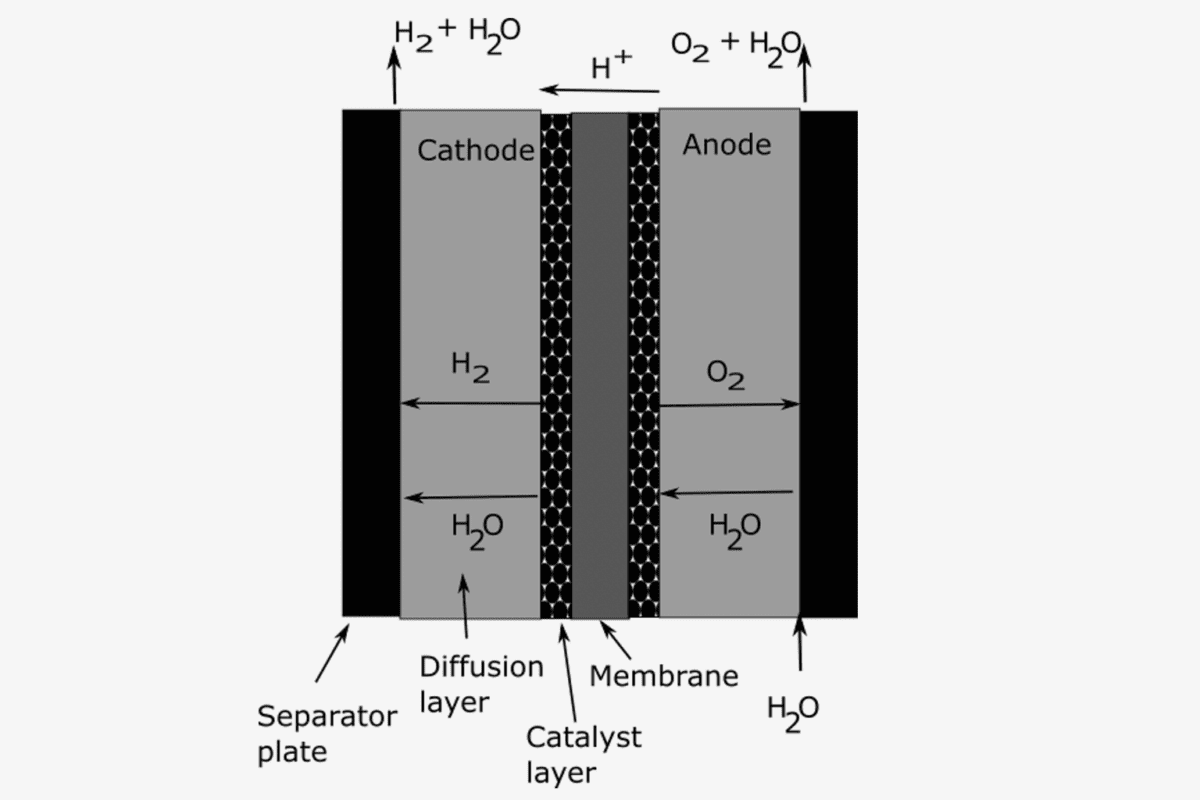
Low-temperature electrolyzers with PEM electrolytes, known as PEM electrolyzers, are promising not only for their higher current density but also for their excellent match with intermittent power sources and the longevity of the commercially offered proton exchange membranes. This enables a compact and durable electrolyzer design.
For high-current density operation at low overpotential1 PEM electrolyzers need rare and expensive of catalysts such as platinum on the cathode side and iridium oxide on the anode side. To realize the potential of PEM electrolyzers, it is crucial that the catalysts are used efficiently and that the catalyst load, i.e. the amount of catalyst per unit area of the electrolyzer cell, is kept to a minimum.
One way of reducing the catalyst load is to deposit the catalyst material on another material known as a catalyst support, either in the form of a thin film or as particles with a diameter of a few nanometers. The catalyst supports act as a scaffolding, allowing the catalyst to be spread over a larger area. An ideal catalyst support should have a large surface area, an open structure that lets water and gases flow to and from the catalyst, excellent contact with the proton exchange membrane and good and stable electrical conductivity to enable the electrochemical reactions. Carbon black is often used as a catalyst support on the cathode side in PEM electrolyzers, but the iridium catalyst on the anode side is generally used without support due to the harsh acidic conditions at the anode.
Carbon nanofibers (CNF) are carbon structures with a diameter that is typically below 100 nm and a length between 1 and 100 µm. Like many carbon nanomaterials, CNF are electrically conductive and mechanically strong.
CNF are grown by chemical vapor deposition (CVD) and have the potential to improve on existing catalyst supports. The CVD growth method makes it possible to control the orientation of the CNF so that they are vertically aligned with a well-defined average spacing, width, and height. This means that the structure of a CNF catalyst support can be adjusted to achieve the large surface area and degree of porosity that is needed.
The structure of a CNF catalyst support also makes it possible to control the position of the catalyst that is deposited on it, which in turn opens possibilities for optimizing the active surface area of the catalyst and reducing the catalyst load. For example, the catalyst can be placed in direct contact or even embedded into the membrane. The CNF can be conformally coated and protected for use on the anode side of the electrolyzer.
Although reducing catalyst load is most important in PEM electrolyzers, CNF catalyst supports may also be used in AEM electrolyzers and in PEM fuel cells. There are clear advantages to using CNF grown by CVD as a catalyst support, such as increasing the active catalyst surface area and decreasing the needed catalyst load. The CVD methods used for CNF production by Smoltek can be used to realize the potential of CNF in electrolysis and fuel cells.
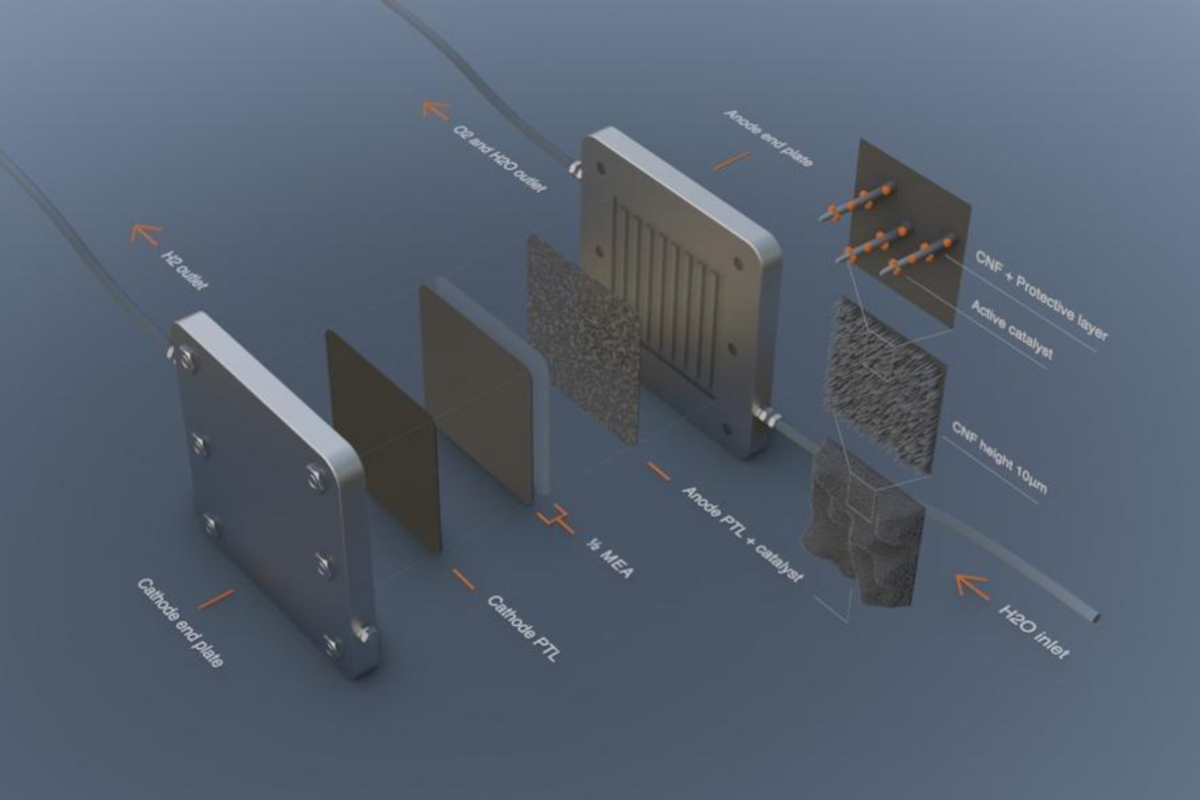
For future needs of the PEM electrolyzer market, when the capacity is scaled to produce Gigawatts of water electrolysis yearly it will be crucial to manage a low iridium catalyst load on PEM anodes to enable cost-efficient hydrogen production.
Smoltek’s nanofiber-based cell materials creates an optimal anode structure that allows iridium catalyst nanoparticles to form a highly active and accessible surface. In principle, all of the nanoparticles come into contact with the proton exchange membrane of the cell potentially reducing the needed amount of iridium by 80% – or more.
Another benefit is that the cells can be optimized for high current density, thus the capacity to produce hydrogen per cell area increases. This is achieved by a corresponding increase of the iridium load. These design choices can create a 2–3 times lower investment cost for the electrolyzer in a hydrogen plant.
Our next step is to industrialize our solution for the electrolyzer cell material (CNF-ECM). We are therefore looking for industrial partner(s) that, in close collaboration with us;
Is your company a potential partner in taking advantage of our disruptive technology?
Contact us today, and let’s arrange a meeting to discuss it further.
Your data will be handled in compliance with our privacy policy.